|
Last Update:
Saturday December 29, 2018
|
| [Home] |
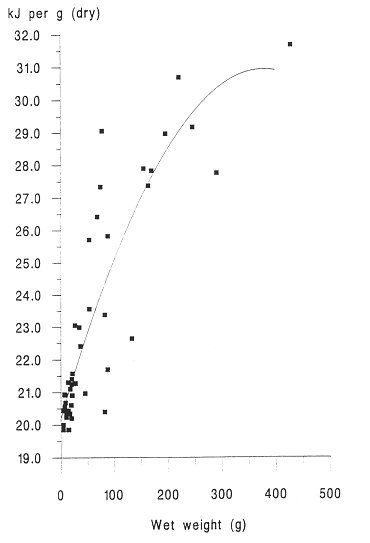
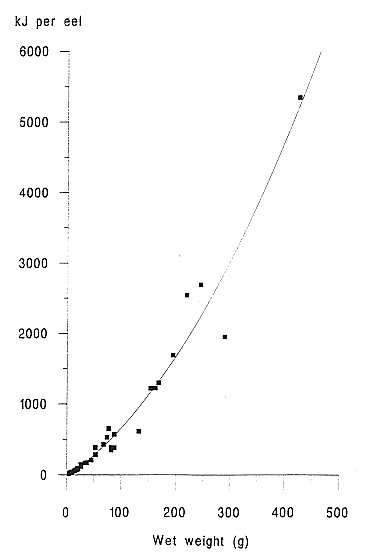
INTRODUCTION One of the most important factors influencing the behaviour and performance of predator species is the nutritional quality of their prey (BEGON et al., 1996). This may be especially important for aquatic carnivores such as otters, because of the thermoregulatory problems of foraging in water and the energy expenses incurred by swimming (KRUUK et al.,1997, KRUUK and CARSS, 1996). To estimate the energetic contents of food from diet analysis, we investigated the variation in energy content with size for eels (Anguilla anguilla), an important prey species of otters throughout northern Europe (KRUUK 1995), common frogs (Rana temporaria) and common toads (Bufo bufo), which are of seasonal importance in the diet of otters in north-east Scotland. We also summarize some existing information on the energetic contents of fishes other than eels. MATERIALS AND METHODS A number of eels were caught by electro-fishing and netting at Lochs Davan and Kinord, Aberdeenshire, Scotland, in the summer of 1995. All the eels were killed by freezing. The wet weight and length of each fish was recorded, and adjusted to allow for reductions of length and weight caused by freezing, using regression equations from SVEDANG and WICKSTROM (1997). Eels were then skinned, the stomachs removed, and then cut into small pieces and freeze dried to constant weight (for eels > 450mm long a sample of post-anal muscle was used, and for smaller individuals the whole eel was used). Samples were then ground to a fine powder using an electric hammer mill, or chopped finely with a sharp knife, and then ground with a mortar and pestle. This procedure was repeated until the sample had been thoroughly homogenised. Samples were stored in airtight vials. Common frogs and common toads were collected during night-time visual searches in the marshes surrounding the lochs. Wet weight and snout-vent length was recorded. We removed the spawn from gravid females (i.e. those containing fully developed eggs) of both species, and the skin of all toads, because these are normally discarded also by otters (WEBER, 1990). Energy content, in kilocalories, of eel and amphibian samples was determined using an adiabatic bomb calorimeter (for procedure see ALLEN, 1974). Approximately 1g of each sample was used for the analysis. Several samples from across the size spectrum of eels and amphibians were duplicated to assess accuracy, which was considered sufficient as the differences in calorific values per gram for the duplicates were never more than 2.5% of the value of the smaller of the two results. Energy contents were expressed as kilojoules (kJ) per animal, kJ per gram (wet weight) and kJ per gram (dry weight). RESULTS A total of 45 eels was analysed. The calorific content per gram (dry weight) ranged between 19.83 kJ/g for the smallest eels to 31.64 kJ/g for the largest (mean = 23.13 kJ/g dry weight, s.e.=0.51, 5.38 kJ/g wet weight, s.e.=0.32). There was a strong and significant positive correlation between energetic content per gram of tissue, and the size of eels expressed as wet weight (Figure 1). The calorific content per gram rises rapidly with the weight of eels up to about 300g, then reaches a maximum. Hence total kJ per eel increased as a quadratic function with the weight of eels (Figure 2). A total of 15 frogs and 15 toads was analysed, with a mean wet body mass for frogs of 29.5g, for toads of 20.7g. The mean calorific values of these amphibians were lower than for eels (frog: 17.84kJ/g dry, s.e. = 0.20, 2.87kJ/g wet, s.e. = 0.13, toad: 17.39kJ/g dry, s.e. = 0.37, 3.42kJ/g wet, s.e. = 0.16 ). Neither frogs nor toads showed significant variation in the energetic content per g of tissue for different sized prey. DISCUSSION The eel results indicated that with increasing size, the energy contents per gram also increased. This is likely to be the result of the increase in the lipid share of the total energy from 20% to 80% during the development from elver to silver eel (BOETIUS and BOETIUS, 1985). DEGANI et al., (1986) also found that total fat content was lower in small eels (39% of dry weight) than in large eels (55% dw), with a rapid increase in percentage body fat for eels up to 200g wet weight, followed by a slower increase for eels of between 200 and 400g, and our results on energetic contents parallel those observations. Our results were similar also to those for the American eel (GALLAGHER et al., 1984). However, in the present study the energetic contents (and presumably, therefore, fat contents) of eels of >400 mm length were considerably less variable than those reported by SVEDANG and WICKSTROM (1997). These data will be useful especially in conjunction with the method developed by CARSS and ELSTON (1996) for the assessment of the size of eels taken by otters, from measurements of vertebrae in spraints. In comparison with eels, the calorific values of amphibians were much lower and there was no increase in calorific value of tissue with the size of animals. SMITH (1950) showed for Rana temporaria that fat content was lowest during the spring breeding season (when they are most common in the diet of otters; WEBER 1990) and it reached a maximum prior to hibernation.
Table 1 shows the results of the present study along with results for other species of fish. The results of the present study suggest that eels represent, in terms of calorific content, a much more profitable prey item for otters than amphibians. Correspondingly, the results of studies on otter diet which have shown that, where present, eels often form a considerable proportion of the diet (CARRS et. al., 1998). During the spring months, however, fish availability is lowest (KRUUK et al. ,1993), and frogs and toads congregate in large numbers to breed. Under these circumstances the high availability and reduced time of capture may make it energetically worthwhile for otters to feed selectively on amphibians despite their lower calorific values. A recent study of otter-amphibian interactions in the north-east of Scotland showed that prey remains in spraints are commonest during the spring (L. BROWN, pers. comm.). Results from the same study have shown that, at this time of year, toads are often heavily predated by otters, despite the fact that the presence of poisonous dermal glands demands the removal of their skin by otters, and a subsequent increase in handling time (WEBER, 1990). SIDOROVICH and PIKULIK (1997) also described otter predation on toads in Belarus, when unusual weather conditions simultaneously reduced the availability of alternative prey and resulted in high densities of toads. Acknowledgements: We are grateful to Dr D.N. Carss and L. Brown for assistance with providing specimens. REFERENCES Allen, S.E. (Ed) (1989). Chemical Analysis of Ecological Materials, Blackwell Scientific Publications, Oxford |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| [Copyright © 2006 - 2050 IUCN/SSC OSG] | [Home] | [Contact Us] |