|
Last Update:
Thursday November 22, 2018
|
| [Home] |
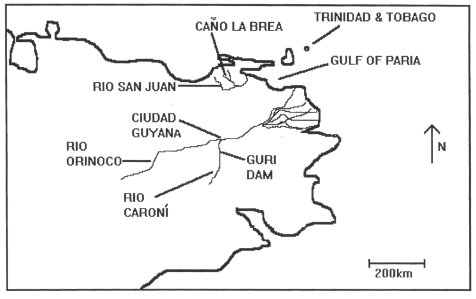
INTRODUCTION The Giant Otter, Pteronura brasiliensis, is the largest member of the family Mustelidae. It formerly inhabited most freshwater streams of South America from Venezuela to Argentina (Eisenberg, 1989). However, by the early 1970s, pelt hunters had decimated many populations leading to its classification as ‘vulnerable’ in the World Conservation Union (IUCN) Mammal Red Data Book (Thornbeck and Jenkins, 1982). A recent review of the Giant Otter’s status (Carter and Rosas, 1997) suggests that it be elevated to that of ‘endangered’. The present distribution of this species has recently been described by Carter and Rosas (1997). This article discusses a population of Giant Otters studied during the dry season of 1994 in Caño la Brea, North-East Venezuela. This area is approximately two hundred kilometers North of the most Northern population they specify which inhabits the Rio Caroni by the Guri Dam. The relative positions of the two populations are shown in Figure 1. This finding greatly increases the species’ known geographic range.
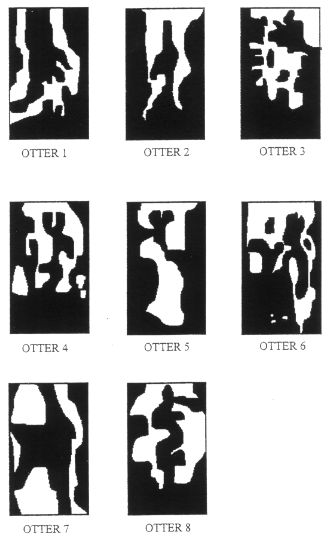
The Caño la Brea river is a tributary of the Rio San Juan in Sucre state, Venezuela. It is 34 kilometers long and varies between 3 and 6 meters in width. It is subjected to tidal movements of brackish water but its upper reaches are consistently fresh. The surrounding river networks contain only brackish water. The first twenty kilometers of the river are surrounded on either side by mangrove forests. Further upstream, it is bordered by mixed scrub, beyond which is open savannah. The presence of Giant Otters in this area is a cause for optimism: there may be other undiscovered populations in the area. However, if this group is as isolated as it appears, there is also cause for concern regarding its survival. SURVEY METHOD Survey work was carried out during the dry season from February until May 1994. Observations of the Giant Otter population were made from canoes. The river was divided into 34 x 1 kilometer sections. Each kilometer was surveyed by paddling slowly along the river for one hour until an otter or group of otters was sighted. At least one, one hour survey was carried out in each kilometer at the following times of day: dawn (about 5.30am) - 7.30am, 7.30am - 10.30am, 10.30am - 4.30pm and 4.30pm - dusk (about 6.30pm). The total study involved 201 hours of survey time. Otters were observed either directly or through binoculars. The number of individuals present at each sighting was recorded as was their location on the river. In addition, drawings were done and photographs taken in order to identify individuals on the basis of their throat-markings (SCHENCK and STAIB, 1995b). An estimate of the total number of animals living in the area was made. Their behaviour was also recorded. RESULTS: THE PRESENCE OF GIANT OTTERS IN THE STUDY AREA The survey resulted in 44 sightings of Giant Otters and 445 minutes of observation. Individual throat markings are easily seen when the animals periscope out of the water. Figure 2. shows the throat marking of each animal that was able to be identified. In addition to these eight fully grown animals, two cubs were seen. A conservative population estimate is therefore 10.
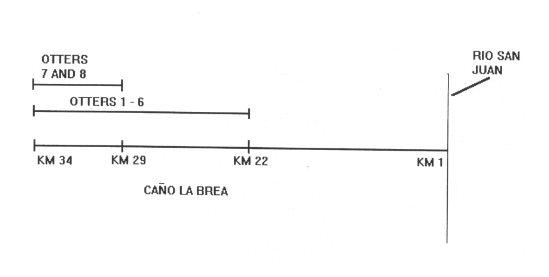
Otters 1 - 6 were commonly seen together. On one occasion, two cubs (their head size half that of the adults) were seen with this group. Otters 7 and 8 were always seen together and never seen with the rest of the group. Their behaviour was discrete and they disappeared quickly. Individuals not living in family groups are referred to as transients and commonly show this type of behaviour (Carter and Rosas, 1997). Giant Otters were seen only between kilometers 22 and 34 of the river. They were sighted throughout the day. DISCUSSION Number Of Individuals Giant otters generally live in groups of 3 - 9 individuals consisting of a mated pair and one or two litters (Carter and Rosas, 1997). They are highly cohesive: group members rest, play, sleep, travel and fish together. Otters 1 - 6 in Caño la Brea make up such a group. They were often observed together and their behaviour further supports this. Constant verbal communication between the group, travelling, nuzzling, grooming and fishing together was all observed. Group defence is co-operative with the female generally taking the front line (SCHENCK and STAIB, 1994). On several occasions, otters 1 - 3 approached the canoe snorting and periscoping while the others would stay at the river bank. Otter 2 was almost always the first to approach thus suggesting it was the dominant female of the group. However, no definitive sex determination of individuals was possible during this study. In environments such as Caño la Brea where there are distinct wet and dry seasons, females tend to give birth during the dry season (Duplaix, 1980; Schweizer, 1992). The two cubs were seen for the first and only time in late April. They were swimming independently rather than being carried and were therefore thought to be about 3 - 4 months old. Cubs do not begin hunting and travelling with the family until they are of this age (Duplaix, 1980; Laidler, 1984). Cubs are carried between holts before this, probably to avoid parasitic infection from extended periods in one holt (Schenck and Staib, 1994). Their presence shows clearly that Caño la Brea provides an excellent habitat for Giant Otters and that the group living in it are reproductively active. Otters 7 and 8 were thought to be transients. They may have been subadults recently split from their family group or adults which have lost a mate (Carter and Rosas, 1997). Such animals lack an established territory and are usually shy and difficult to sight. This was characteristic of these two individuals. They were seen only in the top few kilometers of the river and were sighted much less frequently than the family group. The range of the family group and the transient otters can be seen in Figure 3.
Distribution Within The Study Area This study was carried out during the dry season when the savannah surrounding the river was dry. Giant Otters were seen between kilometers 22 and 34. Downstream of kilometer 22, the clear quality of the water was lost and the salinity rose sharply (pers. obsv.). A clear preference for clear or black water which is reasonably transparent (1.0 - 4.3m) and supports a large diversity of fish is shown by the Giant Otter (Sioli, 1984; Goulding et al., 1988). This seems to explain the observed distribution. A previous ecological study of the area (Project Mermaid, 1992) during the wet season saw Giant Otters on only two occasions using the same sampling regime. At this time, the whole river was clear and the surrounding savannah flooded. Giant Otters are known to move onto flooded forest during the wet season to exploit the influx of fish (Duplaix, 1980; Schweizer, 1992). The dramatically smaller number of sightings during the wet season thus suggests that they increase their home range size during this time. Several Giant Otter campsites (Duplaix, 1980; Laidler, 1984) were found during the study period. These were areas cleared of vegetation at the river edge. Fish scales and broken snail shells were often found and the areas usually had a fishy smell. Two den sites (Mondolfi, 1970; Duplaix, 1980; Schweizer, 1992; Carter and Rosas, 1997) were also found under mangrove roots. CONCLUSIONS This study confirms that Giant Otters occur further North in South America than was previously thought. The group appears to be healthy, numerous and is certainly reproductively active. This is cause for optimism, especially in terms of the possibilities of other undiscovered populations. However, there are many threats that this population face. If they are as isolated as they appear, they may become or may already be inbred. This can cause a decrease in survival, growth or fertility of individuals and more importantly make them increasingly susceptible to disease (CAUGHLEY, 1994). Waro indians in the area come into the area once a year to hunt and fish. They do not hunt the otters but they bring with them many domestic dogs. These could transmit disease to the otters which would spread fast due to the tactile nature of Giant Otter groups. Transmission of disease from domestic to wild stock repeatedly had detrimental consequences (for example, 1.000 Serengeti lions died from canine distemper probably transmitted from domestic dogs, MACDONALD, 1996). SCHENCK and STAIB (1994) report of a young male Giant Otter which stayed close to a village where many cats and dogs were infected with parvovirosis. It then returned to the inner Manu National Park. If the Caño la Brea group is inbred, the consequences of disease transmission could be devastating. REFERENCES Carter, S.K., Rosas, F.C.W. (1997). Biology and Conservation of the Giant Otter Pteronura brasiliensis. Mammal Rev. 27: 1-26. |
| [Copyright © 2006 - 2050 IUCN/SSC OSG] | [Home] | [Contact Us] |